COVID-19 vaccine trials start in area
Participants living in the area are now being accepted to be part of a national COVID-19 vaccine clinical trial coordinated in northeast Louisiana through Monroebased Clinical Trials of America (CTA).
The AstraZeneca pharmaceutical company will be conducting the trials beginning in August while more than 30,000 people have participated in Phase III of testing thus far.
Jeb Andrews, CEO of CTA, said the company is honored to have been selected to secure the 1,500 participants in northeast Louisiana. He said two-thirds of the participants will receive the live vaccine while the remaining receive a placebo.
Andrews noted that typically trials like this one have a 50/50 ratio of vaccine/placebo.
The two-part vaccine trials are open to adults at least 18 years of age who meet the following criteria:
• Are not pregnant or breastfeeding
• Have no history of Guillain Barre Syndrome?
•Have no had previously confirmed SARS-CoV-2 infection
• Have had no cancer in the past two years
Participants in the trial will receive compensation for time and travel endeavors involving testing. The trial is first come, first served, and those interested should text COVID NELA to 31996, or call 318-267-4111.
More information on testing can be found at CTAmerica.net.
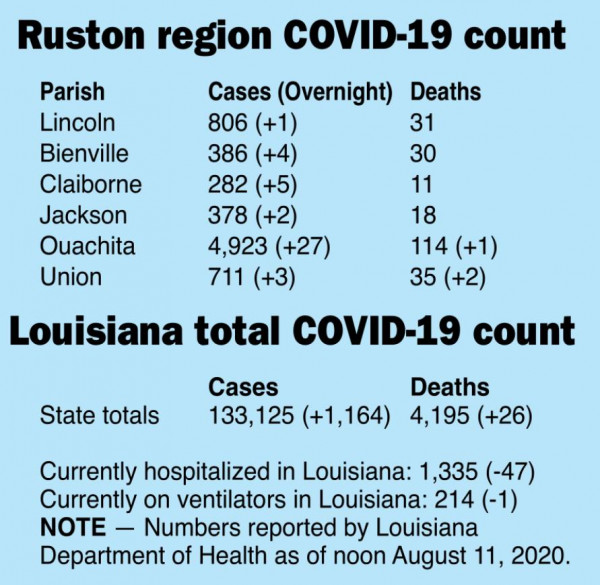
Lincoln Parish Cases
Only one new case of COVID-19 was reported overnight in the daily numbers released by the Louisiana Department of Health on Tuesday.
Statewide, Louisiana reported an overall total of 133,125 COVID-19 cases for an increase of 1,164 since Monday’s numbers were released.
The state COVID-19 death total climbed to 4,195 Tuesday, an increase of 26 since Monday.

